Endometriosi
 | |
| Tipus | malaltia del sistema reproductor femení, endometrial disease (en) |
|---|---|
| Especialitat | ginecologia |
| Clínica-tractament | |
Medicació danazol, 17α-hydroxyprogesterone (en) | |
| Patogènia | |
| Localització | abdomen i pelvis |
| Associació genètica | PDE1C (en) |
| Classificació | |
| CIM-11 | GA10 |
| CIM-10 | N80 i N80.9 |
| CIM-9 | 617, 617.9 i 617.8 |
| CIAP | X99 |
| Recursos externs | |
| Enciclopèdia Catalana | 0102148 |
| OMIM | 131200 |
| DiseasesDB | 4269 |
| MedlinePlus | 000915 |
| eMedicine | 271899 i 795771 |
| Patient UK | endometriosis-pro |
| MeSH | D004715 |
| Orphanet | 137820 |
| UMLS CUI | C0014175, C0014175, C0269102 i C0404545 |
| DOID | DOID:289 |
L'endometriosi (del grec: ἔνδον endon (interior); μήτρα mētra (matriu, úter); ωσις, osis (procés patològic); lit. procés patològic de l'interior de l'úter) és una condició en què cèl·lules semblants a les de l'endometri (la capa de teixit que normalment cobreix l'interior de l'úter) creixen fora de l'úter.[1][2] Molt sovint succeeix als ovaris, a les trompes de Fal·lopi i al teixit al voltant de l'úter i els ovaris; però, en casos excepcionals, també pot ocórrer en altres parts del cos.[3] Els símptomes principals són el dolor pelvià i la infertilitat.[4] Gairebé la meitat de les afectades tenen dolor pelvià crònic, mentre que en el 70% dels casos es produeix dolor durant la menstruació.[4] També es freqüent el dolor durant les relacions sexuals.[4] La infertilitat es produeix fins a la meitat de les dones afectades.[4] Els símptomes menys freqüents inclouen símptomes urinaris o intestinals.[4] Al voltant del 25% de les dones no presenten símptomes.[4] L'endometriosi pot tenir efectes tant socials com psicològics.[5]
La causa no està del tot clara.[4] Els factors de risc inclouen tenir antecedents familiars amb aquesta malaltia.[3] Les àrees afectades per l'endometriosi sagnen cada mes, provocant inflamacions i cicatrius.[3][4] Els creixements causats per l'endometriosi no són càncer, però hi ha una associació entre endometriosi i determinats tipus de càncer.[3] El diagnòstic generalment es basa en símptomes en combinació amb imatges mèdiques,[3] però la biòpsia és el mètode de diagnòstic més segur.[3] Altres causes amb símptomes semblants inclouen la malaltia inflamatòria pelviana, la síndrome de l'intestí irritable, la cistitis intersticial i la fibromialgia.[4]
L'evidència suggereix que l'ús de la píndola anticonceptiva redueix el risc d'endometriosi.[6] També poden ser preventius l'exercici i evitar grans quantitats d'alcohol.[3] No hi ha cura per l'endometriosi, però una sèrie de tractaments poden millorar els símptomes.[4] Això pot incloure medicaments per al dolor, tractaments hormonals o cirurgia.[3] La medicació recomanada per a disminuir el dolor sol ser un fàrmac antiinflamatori no esteroidal (AINE), com el naproxén.[3] També pot ser útil prendre contínuament el component actiu de la píndola anticonceptiva o utilitzar un dispositiu intrauterí amb progestògen.[3] L'agonista de l'hormona que allibera gonadotropina (GnRH agonista) pot millorar la capacitat d'aquelles que són infèrtils per quedar embarassades.[3] L'eliminació quirúrgica de l'endometriosi es pot utilitzar per tractar aquelles afectades que els seus símptomes no són manejables amb altres tractaments.[3]
L'endometriosi es va determinar per primera vegada com una condició separada a la dècada del 1920;[7] abans d'aquesta època, es considerava que l'endometriosi i l'adenomiosi eren la mateixa malaltia.[7] No està clar qui va descriure per primera vegada la malaltia.[7]
Història[modifica]
L'endometriosi va ser descoberta microscòpicament per Karl von Rokitansky el 1860,[8] encara que ja va ser documentada en textos mèdics fa més de 4.000 anys.[9] El corpus hipocràtic presenta símptomes similars a l'endometriosi, incloent úlceres uterines, adhesions i infertilitat.[9]
Històricament, les dones amb aquests símptomes van ser tractades amb sangoneres, camises de força, sagnies, dutxes vaginals, mutilacions genitals, embaràs (com a forma de tractament), penjant-les de cap per avall, intervenció quirúrgica, i fins i tot matant-les quan es sospitava d'una possessió diabòlica.[9] Els metges hipocràtics van reconèixer i tractar el dolor pelvià crònic com un autèntic desordre orgànic fa 2.500 anys, però durant l'edat mitjana es va produir el canvi cap a la creença que les dones amb dolor pelvià estaven boges, eren immorals, s'imaginaven el dolor, o simplement eren dolentes;[9] sovint, els símptomes del dolor pelvià crònic inexplicable es van atribuir a la imaginació d'una boja, la debilitat femenina, la promiscuïtat o la histèria.[9] Possiblement, molts casos d'endrometriosi van ser diagnosticats com a histèria, que es pensava que era una malaltia mental.[9] La idea que el dolor pelvià crònic estava relacionat amb la malaltia mental va influir en les actituds modernes respecte a les dones amb endometriosi, el que va provocar retards en el diagnòstic correcte i la indiferència al veritable dolor de la pacient durant el segle xx.[9]
Els metges hipocràtics creien que retardar la fecundació podria provocar malalties de l'úter, causant símptomes semblants a l'endometriosi. Es va animar a les dones amb dismenorrea a casar-se i tenir fills a una edat molt jove.[9] El fet que els hipocràtics recomanessin canvis en les pràctiques matrimonials a causa de malalties semblants a l'endometriosi implica que aquesta malaltia probablement fos comuna, amb taxes superiors a la prevalença del 5-15% que sovint se cita actualment.[9] Si, efectivament, aquest trastorn era tan comú històricament, això pot influenciar a les teories modernes que suggereixen vincles entre endometriosi i les dioxines, PCB i productes químics.[9]
Epidemiologia[modifica]
Al 2015 es va estimar que estaven afectades 10,8 milions de dones de tot el món.[10] Altres fonts estimen que estan afectades entre el 6 i 10% de les dones.[4] Poden estar afectades més de l'11% de les dones estatunidenques d'entre 15 i 44 anys.[3] L'endometriosi és més freqüent entre 30 i 40 anys; no obstant això, pot començar en les nenes a partir dels 8 anys.[3][11]
Pot afectar qualsevol dona, des de la premenarquia fins a la postmenopausa, independentment de la raça o l'ètnia o si han tingut fills o no. Es tracta principalment d'una malaltia dels anys reproductius.[12] S'han produït incidències d'endometriosi en dones postmenopàusiques[13] i, en casos menys freqüents, les nenes poden presentar símptomes d'endometriosi abans d'arribar a la menarquia.[14][15]
L'endometriosi produeix poques morts (~100 el 2015).[16]
Senyals i símptomes[modifica]
El dolor pelvià i la infertilitat són símptomes comuns, tot i que el 20-25% de les dones són asimptomàtiques.[4]
Dolor pelvià[modifica]

Un símptoma important de l'endometriosi és el dolor pelvià recurrent. El dolor pot variar entre un espasme muscular lleu o greu o un dolor progressiu que es produeix en els dos costats de la pelvis, a la part baixa de l'esquena, a la zona rectal, i fins i tot per les cames. La quantitat de dolor que se sent una dona es correlaciona feblement amb l'extensió o l'etapa (de l'1 al 4) d'endometriosi, amb algunes dones que pateixen poc o cap dolor tot i tenir una endometriosi extensa o una endometriosi amb cicatrius, mentre que altres dones poden tenir un dolor sever encara que tinguin només unes petites àrees d'endometriosi.[17] Els símptomes del dolor relacionat amb l'endometriosi poden incloure:
- dismenorrea: dolorosa i, de vegades, amb rampes incapacitants durant el període menstrual; el dolor pot empitjorar amb el temps (dolor progressiu), també apareixen dolors a la part inferior de l'esquena relacionades amb la pelvis.
- dolor pelvià crònic: típicament acompanyat de mal d'esquena o dolor abdominal.
- disparèunia: sexe dolorós.
- disúria: micció urgent, freqüent i, de vegades, dolorosa.
En comparació amb les dones amb endometriosi superficial, les que tenen una malaltia profunda semblen més propenses a patir el dolor rectal, i fins i tot tenir la sensació que les seves entranyes són expulsades.[18] Les àrees de dolor individuals i la intensitat del dolor no semblen estar relacionades amb el diagnòstic quirúrgic, i la zona de dolor no està relacionada amb l'àrea afectada per l'endometriosi.[18]
Hi ha diverses causes de dolor. Les lesions d'endometriosi reaccionen a l'estimulació hormonal i poden «sagnar» en el moment de la menstruació. La sang s'acumula localment si no és netejada en breu pel sistema immunitari, circulatori i limfàtic. Això pot conduir a la inflamació, que desencadena l'activació de citocines, que causa dolor. Una altra font de dolor és la dislocació dels òrgans, que sorgeix quan els òrgans interns s'uneixen per adhesió entre ells (es poden unir els ovaris, l'úter, els oviductes, el peritoneu i la bufeta); el dolor provocat d'aquesta manera pot durar tot el cicle menstrual, no només durant els períodes de menstruació.[19]
A més, les lesions endometriòtiques poden desenvolupar el seu propi subministrament nerviós, creant així una interacció directa i bidireccional entre les lesions i el sistema nerviós central, que pot produir una varietat de diferències individuals en el dolor que poden, en algunes dones, independitzar-se de la pròpia malaltia.[17] Es creu que les fibres nervioses i els vasos sanguinis creixen en lesions d'endometriosi mitjançant un procés conegut com a neuroangiogènesi.[20]
Infertilitat[modifica]
Al voltant d'un terç de les dones amb infertilitat tenen endometriosi.[4] Entre les dones amb endometriosi, al voltant del 40% són infèrtils.[4] La patogènia de la infertilitat depèn de l'etapa de la malaltia; en l'etapa inicial de la malaltia es planteja la hipòtesi que això és secundari a una resposta inflamatòria que afecta diversos aspectes de la concepció, mentre que en una etapa posterior la anatomia i l'adhesió pelviana distorsionades de la malaltia contribueixen a l'alteració de la fecundació.[21]
Altes símptomes[modifica]
Altres símptomes inclouen diarrea o restrenyiment,[18] fatiga crònica, nàusees i vòmits, mals de cap, una mica de febre, períodes pesats i / o irregulars, i hipoglucèmia.[22][23]
A més del dolor durant la menstruació, el dolor de l'endometriosi pot ocórrer en altres moments del mes. Pot haver dolor durant l'ovulació, dolor associat amb adherències, dolor causat per la inflamació a la cavitat de la pelvis, dolor durant els moviments intestinals i la micció, dolor durant el moviment corporal general com l'exercici, estar dret o caminar, i el dolor amb el coit. El dolor més greu sol associar-se a la menstruació. El dolor també pot començar una setmana abans d'un període menstrual, durant i fins i tot una setmana després d'un període menstrual, o pot ser constant. El dolor pot ser debilitant i pot causar estrès emocional.[24]
Hi ha una associació entre l'endometriosi i determinats tipus de càncer, en particular alguns tipus de càncer d'ovari,[25][26] limfoma no hodgkinià i tumor cerebral.[27] L'endometriosi no està relacionada amb el càncer d'endometri.[28]
Factors de risc[modifica]
La causa no està del tot clara.[4] Els factors de risc inclouen tenir un antecedent familiar de la malaltia.[3]
Factors genètics[modifica]
La predisposició genètica té un paper important.[29] Les filles o germanes de dones amb endometriosi tenen un major risc de desenvolupar l'endometriosi; els nivells baixos de progesterona poden ser genètics, i poden contribuir a un desequilibri hormonal.[30] Hi ha una incidència de sis vegades més gran en dones amb una familiar afectada de primer grau.[31]
S'ha proposat que l'endometriosi prové d'una sèrie d'impactes múltiples en els gens diana, en un mecanisme similar al desenvolupament del càncer. En aquest cas, la mutació inicial pot ser somàtica o hereditària.[29]
Els canvis individuals del genoma (que es troba mitjançant la genotipificació, inclosos els estudis d'associació del genoma complet) que s'han associat amb l'endometriosi, inclouen:
| Cromosoma | Gen/Regió de la mutuació | Producte genètic | Funció |
|---|---|---|---|
| 1 | WNT4[32] | Membre de la família sense lloc de integració de MMTV 4 | Vital per al desenvolupament dels òrgans reproductors femenins |
| 2 | GREB1/FN1[32] | Regulació del creixement per estrogens del càncer de mama 1/Fibronectí 1 | Gen de resposta precoç en la via de regulació d'estrògens / Processos d'adhesió i migració cel·lular |
| 6 | ID4[32] | Inhibidor de la unió de l'ADN 4 | Oncogen ovàric, funció biològica desconeguda |
| 7 | 7p15.2[32][33] | Factors de transcripció | Influència de la regulació transcripcional del desenvolupament uterí |
| 9 | CDKN2BAS[32] | Inhibidor de quinasa dependent de la ciclina 2B ARN sentit contrari | Regulació dels gens supressors de tumors |
| 10 | 10q26[34] | ||
| 12 | VEZT[32] | Vezatina, una proteïna d'unió adherida a la transmembrana | Gen supressor de tumors |
| 19 | MUC16 (CA-125) | Mucina 16, associat a la superfície cel·lular | Forma barreres de mucosa protectora |
A més, hi ha una associació més feble amb els canvis en el gen de la fibronectina, així com en la regió 2p14 del cromosoma 2.[32]
Hi ha moltes troballes d'expressió gènica alterada i epigenètica, però ambdós també poden ser un resultat secundari de, per exemple, factors ambientals i metabolisme alterat. Exemples d'expressió gènica alterada inclouen el de miRNAs.[29]
Altres factors[modifica]
Alguns factors associats amb l'endometriosi inclouen:
- no haver donat a llum (nul·liparitat).[35]
- exposició perllongada als estrògens; per exemple, en la menopausa tardana[36] o en la menarquia precoç.[37][38]
- obstrucció de la sortida menstrual; per exemple, en anomalies de Müllerian.[36]
Les toxines ambientals[modifica]
Diversos estudis han investigat el vincle potencial entre l'exposició a les dioxines i l'endometriosi, però l'evidència no es prou clara i els mecanismes potencials són poc entesos.[39] Una revisió de 2004 sobre estudis de dioxines i endometriosi va concloure que «les dades humanes que donen suport a l'associació de dioxines-endometriosis són escasses i conflictives»,[40] i una revisió de seguiment de 2009 també va trobar que hi havia «proves insuficients» en suport d'un vincle entre l'exposició a la dioxina i les dones que desenvolupen endometriosi.[41] Una revisió de 2008 va concloure que es necessitava més treball, afirmant que «encara que el treball preliminar suggereix una implicació potencial de l'exposició a les dioxines en la patogènesi de l'endometriosi, queda molt per definir clarament la causa i l'efecte i comprendre el possible mecanisme de toxicitat».[42]
Fisiopatologia[modifica]

Encara que la causa exacta de l'endometriosi roman desconeguda, s'han presentat moltes teories per comprendre millor i explicar el seu desenvolupament. Aquests conceptes no s'exclouen necessàriament entre si. La fisiopatologia de l'endometriosi probablement és multifactorial i implicarà una interacció entre diversos factors.[29]
Formació[modifica]
Les principals teories per a la formació de l'endometri ectòpic són la menstruació retrògrada, la Müllerianosi, la metaplàsia celòmica i el trasplantament, que es detallen a continuació.
Teoria de la menstruació retrògrada[modifica]
La teoria de la menstruació retrògrada (també anomenada teoria de la implantació o teoria del trasplantament)[43] és la teoria més antiga per a la formació d'endometri ectòpic en l'endometriosi.[29] Sugereix que durant el fluix menstrual d'una dona, alguns residus endometrials flueixen cap enrere a través de les trompes de Fal·lopi i cap a la cavitat peritoneal, unint-se a la superfície peritoneal (el revestiment de la cavitat abdominal) on pot procedir a envair el teixit com en forma d'endometriosi.[29]
La menstruació retrògrada sola no és capaç d'explicar tots els casos d'endometriosi, i cal invocar factors addicionals com les diferències genètiques o immunes per explicar el fet que moltes dones amb menstruació retrògrada no tenen endometriosi. A més, l'endometriosi s'ha mostrat en persones que mai han experimentat la menstruació, incloent homes,[44] fetus[45] i noies prepubescentes.[46][15] Més detraccions de la teoria de la menstruació retrògrada són casos d'endometriosi que apareixen al cervell[47] i als pulmons.[48] Aquesta teoria té molts altres problemes associats.[49]
Els investigadors investiguen la possibilitat que el sistema immunitari no pugui fer front a l'atac cíclic del líquid menstrual retrògrad. En aquest context, hi ha interès per estudiar la relació de l'endometriosi amb la malaltia autoinmunitària, les reaccions al·lèrgiques i l'impacte dels materials tòxics.[50][51] Encara no està clar què, si s'escau, existeixi un relació causal entre materials tòxics, malalties autoimmunitàries i endometriosi. Hi ha canvis en el sistema immune en dones amb endometriosi, com ara un augment de productes de secreció derivats de macròfags, però es desconeix si aquests estan contribuint al trastorn o són reaccions d'aquest.[52]
A més, almenys un estudi va trobar que les lesions endometrióticas difereixen en la seva bioquímica a partir de teixits ectòpics transplantats artificialment.[53] Això és probable perquè les cèl·lules que donen lloc a l'endometriosi són una població lateral de cèl·lules.[29] De la mateixa manera, hi ha canvis en, per exemple, el mesoteli del peritoneu en dones amb endometriosi, com la pèrdua d'unions estretes, però es desconeix si són causes o efectes del trastorn.[52]
En casos excepcionals en què l'himen imperforat no es resol abans del primer cicle menstrual i no es detecta, la sang i l'endometri queden atrapats dins de l'úter de la dona fins que el problema es resol per la incisió quirúrgica. Molts metges no es troben amb aquest defecte i, a causa que els símptomes són semblants al de la grip, sovint es diagnostica malament o es passa per alt fins que s'han superat diversos cicles menstruals. Quan finalment es fa un diagnòstic correcte, l'endometri i altres fluids ja han omplert l'úter i les trompes de Fal·lopi amb resultats semblants a la menstruació retrògrada que produeix l'endometriosi. L'estadi inicial de l'endometriosi pot variar en funció del temps transcorregut entre l'inici de la primera menstruació i el procediment quirúrgic.
El primer a proposar la teoria de la menstruació retrògrada com a causa de l'endometriosi va ser John A. Sampson.
Altres teories[modifica]
- Cèl·lules mare: pot sorgir endometriosi a partir de cèl·lules mare de la medul·la òssia, i potencialment d'altres fonts. En particular, aquesta teoria explica l'endometriosi que es troba en zones allunyades de la pelvis, com el cervell o els pulmons.[54]
- Medi ambient: les toxines ambientals (per exemple, la dioxina, el níquel) poden causar endometriosi.[55][56]
- Müllerianosis: una teoria recolzada per l'autòpsia fetal és que les cèl·lules amb potencial per convertir-se en endometri, que es troben establertes en tractes durant el desenvolupament embrional, anomenat «tracte reproductiu femení» (Müllerian), a mesura que migra cap avall a les 8-10 setmanes de vida embrionària podrien dislocar-se de l'úter migratori i actuar com a llavors o cèl·lules mare.[57]
- Metaplàsia celòmica: les cèl·lules celòmiques, que són les avantpassades comunes de les cèl·lules endometrials i peritoneals, poden experimentar metaplàsia (transformació d'un tipus de cèl·lula a l'altra), potser provocada per la inflamació.[58]
- Vasculogènesi: fins a un 37% de l'endoteli microvascular del teixit endometrial ectòpic s'origina a partir de cèl·lules progenitores endotelials (EPC), que donen com a resultat la formació de microorganismes pel procés de vasculogènesi més que no pas el procés convencional d'angiogènesi.[59]
- Creixement neuronal: es troba una major expressió de les fibres nervioses noves en l'endometriosi, però no explica de forma completa la formació del teixit endometrial ectòpic i no es correlaciona definitivament amb la quantitat de dolor percebut.[60]
- Autoimmunitat: La malaltia de Graves és una malaltia autoimmunitària caracteritzada per hipertiroïdisme, goll, oftalmopatia i dermopatia. Les dones amb endometriosi tenien taxes més altes de malaltia de Graves. Un d'aquests possibles vincles entre la malaltia de Graves i l'endometriosi és l'autoimmunitat.[61][62]
Localització[modifica]
Molt sovint, l'endometriosi es troba a:
- ovaris - 52%
- trompes de Fal·lopi - 2-8%
- teixits que mantenen l'úter en el seu lloc (lligaments) - 60%
- superfície exterior de l'úter[3] - 28%
Els llocs menys comuns són:
- vagina
- cèrvix
- vulva
- tracte gastrointestinal
- bufeta urinària - 15%
- recte[3] - 12%
- apèndix vermiforme - 2%
Rarament, l'endometriosi apareix en altres parts del cos, com ara els pulmons, el cervell i la pell.[3]
L'endometriosi rectal o intestinal afecta aproximadament entre el 5 i el 12% de les dones amb endometriosi i pot causar dolor intens amb moviments intestinals.[63]
L'endometriosi es pot estendre al coll uterí i la vagina, o als llocs d'una incisió abdominal quirúrgica, coneguda com a «endometriosi cicatricial».[64] Els factors de risc per l'endometriosi cicatricial inclouen cirurgies abdominals anteriors, com ara una histerectomia o cesària, o embarassos ectòpics, salpingostomia, esterilització puerperal, laparoscòpia, amniocentesi, apendicectomia, episiotomia, histerectomia vaginal i reparació d'hèrnia.[65][66][67]
L'endometriosi també pot presentar-se amb lesions cutànies en endometriosi cutània.
Menys freqüentment es poden trobar lesions en el diafragma. L'endometriosi diafragmàtica és rara, gairebé sempre a l'hemidiafragma dret, i pot infligir el dolor cíclic de l'espatlla dreta just abans i durant un període menstrual. Rarament, l'endometriosi pot ser extraperitoneal, i es troba en els pulmons i al sistema nerviós central.[68]
Diagnòstic[modifica]
Un historial clínic i un examen físic poden portar al metge a sospitar de l'endometriosi. Encara que els metges solen sentir els creixements endometrials durant un examen pelvià, i aquests símptomes poden ser signes d'endometriosi, el diagnòstic no es pot confirmar només mitjançant l'examen.
Al Regne Unit, hi ha una mitjana de 7,5 anys entre que una dona fa la primera visita al metge pels símptomes fins que rep un diagnòstic ferm.[69]
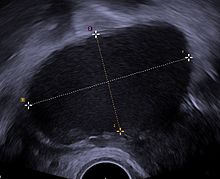
Ultrasò vaginal[modifica]
L'ús de l'ecografia pelviana pot identificar grans quists endometriòtics (anomenats endometriomes). No obstant això, els implants d'endometriosi més petits no es poden visualitzar amb la tècnica d'ecografia.
L'ecografia vaginal té un valor clínic en el diagnòstic de l'endometrioma i abans d'operar una endometriosi profunda.[70] Això s'aplica a la identificació de la propagació de la malaltia en dones amb una sospita clínica ben establerta d'endometriosi.[70] L'ecografia vaginal és econòmica, de fàcil accés, no té contraindicacions i no requereix preparació.[70] Els professionals de la salut que realitzin les ecografies han de tenir experiència.[70] A l'estendre l'avaluació de l'ecografia als compartiments pelvians posteriors i posteriors, el professional de la salut pot avaluar la mobilitat estructural i buscar nòduls endometriòtics profunds i infiltrats i pendre nota de la mida, la ubicació i la distància de l'anus, si escau.[71] La detecció amb ecografia d'una endometriosi profunda no només reduirà la quantitat de laparoscopies diagnòstiques, sinó que guiarà la gestió i millorarà la qualitat de vida.[71]
Laparoscòpia[modifica]

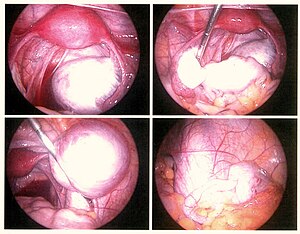
La laparoscòpia, un procediment quirúrgic on s'utilitza una càmera per mirar a l'interior de la cavitat abdominal, és l'única forma de diagnosticar oficialment l'endometriosi, ja que permet la visualització de lesions a menys que la lesió sigui visible externament (per exemple, un nòdul endometriòtic a la vagina). Si els creixements no són visibles, es pot prendre una biòpsia per determinar el diagnòstic.[72] La cirurgia per al diagnòstic també permet alhora el tractament quirúrgic de l'endometriosi.
A l'ull, les lesions poden aparèixer de color blau fosc, negre com la pols de la cendra, vermell, blanc, groc, marró, o no pigmentat. Les lesions varien en grandària. Algunes dins de les parets de la pelvis poden no ser visibles, ja que el peritoneu normal de les dones infèrtils revela l'endometriosi en la biòpsia en un 6-13% dels casos.[73] L'endometriosi precoç normalment es produeix a les superfícies dels òrgans a les zones pelviana i intraabdominal. Els professionals sanitaris poden anomenar les àrees d'endometriosi per diferents noms, com implants, lesions o nòduls. Es poden observar lesions més grans en els ovaris, com ara endometriomes o «quists de xocolata», «xocolata», ja que contenen un fluid de color marró, majoritàriament de sang quallada.
Freqüentment, durant la laparoscòpia diagnòstica, no es detecten lesions en dones amb dolor pelvià crònic, símptoma comú a altres trastorns, incloent adenomiosi, adhesions pelvianes, malaltia inflamatòria pelviana, anomalies congènites del tracte reproductor, i masses ovàriques o trompes.[74]
Etapes[modifica]
Per diagnosticar l'endometriosi amb exactitud, els metges generalment utilitzen un sistema de quatre etapes (I-IV) desenvolupat per la Societat Americana de Medicina Reproductiva (American Society for Reproductive Medicine, ASRM) el 1997.[75] El procés és un sistema complex de punts que valora lesions i adhesions en els òrgans pelvians, però és important tenir en compte que les etapes només avaluen la malaltia física, no el nivell de dolor o infertilitat. Una persona amb endometriosi en etapa I pot tenir una mica de malaltia i dolor intens, mentre que una persona amb endometriosi en etapa IV pot tenir malaltia greu i sense dolor, o viceversa. En principi, les diferents etapes mostren aquestes característiques:
- Etapa I (endometriosi mínima): es caracteritza per la presència de lesions petites i superficials en els implants endometriòtics a l'ovari. Aquests implants sovint estan aïllats i no mostren adherències significatives o teixit cicatricial que s'assembli a teranyines fines o denses. Les persones amb endometriosi en etapa I també pateixen inflamació en els òrgans de la cavitat pelviana.
- Etapa II (endometriosi lleu): les pacients amb endometriosi en etapa II generalment tenen lesions lleugeres i implants superficials en un ovari i en el recobriment pelvià. Aquests implants superficials tenen fama de ser menors a 5 centímetres en total i no tenen adherències significatives.
- Etapa III (endometriosis moderada): hi ha implants profunds en l'ovari i en el recobriment pelvià. En aquesta etapa, es poden observar més lesions i ja hi ha evidències d'implants i adherències múltiples al voltant de les trompes i els ovaris. És important tractar-se el més aviat possible, especialment si planeja concebre en el futur, ja que la cicatrització podria dificultar eventualment l'entrada de l'òvul a la trompa de Fal·lopi.
- Etapa IV (endometriosis greu): és l'etapa final de endometriosi. Els implants profunds en el recobriment pelvià i els ovaris ja són visibles, i es podrien materialitzar lesions en les trompes de Fal·lopi i l'intestí. Són visibles múltiples implants que involucren adherències i endometriomes ovàrics o quists ovàrics grans. L'endometriosi en etapa IV també pot ocasionar el bloqueig de les trompes de Fal·lopi i el dany dels ovaris. Les dones amb aquest problema generalment tenen dificultats per quedar-se embarassades i sovint requereixen un tractament avançat de fertilitat.
A més d'aquestes característiques distintives que diferencien cada etapa, la ubicació, quantitat, profunditat i grandària del teixit endometrial representa un paper en la determinació de l'etapa d'un cas d'endometriosi.
Biomarcadors[modifica]
Una àrea d'investigació és la recerca de biomarcadors d'endometriosi.[76]
El 2010, essencialment, tots els biomarcadors proposats per l'endometriosi van ser d'ús mèdic poc clar, encara que alguns semblen prometedors.[76] L'únic biomarcador que s'ha utilitzat durant els últims vint anys és el CA-125.[76] Una revisió de 2016 va trobar que en aquelles dones amb símptomes d'endometriosi, i una vegada que s'ha descartat el càncer d'ovari, un CA-125 positiu pot confirmar el diagnòstic.[77] Però el seu acompliment en descartar l'endometriosi és baixa.[77] Els nivells de CA-125 semblen caure durant el tractament d'endometriosi, però no han mostrat una correlació amb la resposta de la malaltia.[76]
Una altra revisió del 2011 va identificar diversos biomarcadors putatius sobre la biòpsia, incloent troballes de petites fibres nervioses sensorials o una subunitat d'integrina β3 defectuosa.[78] Es va postular que una futura eina de diagnòstic per a l'endometriosi consistirà en un panell de diversos biomarcadors específics i sensibles, que inclouen tant les concentracions de substàncies com la predisposició genètica.[76]
Histopatologia[modifica]



Les lesions endometriòtiques típiques mostren característiques histopatològiques similars a l'endometri, anomenat estroma endometrial, l'epiteli endometrial i les glàndules que responen als estímuls hormonals. Les lesions més grans no poden mostrar cap glàndula, sinó dipòsits externs (vegeu la micrografia de la dreta) com a residual.
S'ha trobat que la immunohistoquímica és útil en el diagnòstic de l'endometriosi, ja que les cèl·lules estromals tenen un antigen de superfície especial, el CD10, permetent així que el patòleg es dirigeixi directament a una zona de tinció i, per tant, confirmi la presència de cèl·lules estromals i identificar el teixit glandular tenyit amb la rutinària tinció d'HE.[79]
Quantificació del dolor[modifica]
L'escala de dolor més comuna per quantificar el dolor relacionat amb endometriosi és l'escala visual analògica (EVA) (Visual Analogue Scale, VAS); l'EVA i l'escala de qualificació numèrica (Numerical Rating Scale, NRS) van ser les millors escales de dolor adequades per al mesurament del dolor en l'endometriosi. A efectes d'investigació, i per al mesurament del dolor més detallat en la pràctica clínica, l'EVA o el NRS per cada tipus de dolor típic relacionat amb l'endometriosi (dismenorrea, disparèunia profunda i dolor pelvià crònic no menstrual) s'utilitzen en combinació amb la impressió global clínica (Clinical Global Imperssion, CGI) i una escala de qualitat de vida.[80]
Prevenció[modifica]
L'evidència limitada indica que l'ús d'anticonceptius orals combinats està associat a un risc reduït d'endometriosi.[6]
Tractament[modifica]
Tractament nutricional[modifica]
A més dels tractaments mèdics convencionals, s'ha reconegut que la nutrició pot exercir un paper important en el maneig dels símptomes de l'endometriosi.
El tractament nutricional per a l'endometriosi se centra en una dieta equilibrada i saludable que puga ajudar a reduir la inflamació en el cos i alleujar els símptomes dolorosos associats amb aquesta condició. S'ha observat que uns certs aliments i nutrients poden influir positivament en el maneig de l'endometriosi:
- Dieta rica en fibres: Es recomana consumir una dieta alta en fibra, que incloga fruites, verdures, llegums i grans sencers. La fibra pot ajudar a regular el sistema digestiu i reduir la inflamació en el cos.
- Àcids grassos omega-3: Els àcids grassos omega-3, presents en anous, xia i llavors de llinosa, tenen propietats antiinflamatòries i poden ajudar a reduir la inflamació associada amb l'endometriosi.
- Antioxidants: Els antioxidants, presents en fruites i verdures acolorides, poden ajudar a reduir l'estrés oxidatiu i la inflamació. Es recomana consumir aliments rics en vitamina C, vitamina E i betacarotens.
- Limitar el consum d'aliments inflamatoris: Reduir la ingesta d'aliments que poden augmentar la inflamació, com la carn roja, els productes lactis rics en greix i els aliments processats, pot ser beneficiós.
- Magnesi: Algunes investigacions suggereixen que el magnesi pot ajudar a alleujar les enrampades menstruals i el dolor associat amb l'endometriosi. Es troba en aliments com a anous, llegums, espinacs i alvocats.
- Evitar cafeïna i alcohol: La cafeïna i l'alcohol poden augmentar els nivells d'estrogen en el cos, la qual cosa pot empitjorar els símptomes de l'endometriosi. Es recomana limitar el seu consum.
És important tindre en compte que la resposta de cada individu a la dieta pot variar, i és essencial consultar a un professional de la salut, com un dietista registrat o un metge especialitzat, per a rebre recomanacions personalitzades en el maneig nutricional de l'endometriosi. A més, el tractament nutricional ha de complementar-se amb un enfocament integral que incloga tractament mèdic, exercici i maneig de l'estrés per a un maneig òptim d'aquesta condició.
Cirurgia[modifica]
Si bé no hi ha cap cura per l'endometriosi, hi ha dos tipus d'intervencions: el tractament del dolor i el tractament de la infertilitat associada a endometriosi.[81] En moltes dones, la menopausa (natural o quirúrgica) redueix el procés.[82] En les dones en els anys reproductius, l'endometriosi només es gestiona; l'objectiu és proporcionar alleujament del dolor, restringir la progressió del procés i restablir o preservar la fertilitat quan sigui necessari. En les dones més joves, el tractament quirúrgic intenta eliminar el teixit endometrial i preservar els ovaris sense danyar el teixit normal.[83]
En general, el diagnòstic de l'endometriosi es confirma durant la cirurgia, moment en què es poden prendre passos ablatius. Altres passos depenen de les circumstàncies; una dona sense infertilitat es pot tractar amb medicaments hormonals que suprimeixen el cicle natural i la medicació per al dolor, mentre que una dona infèrtil es pot tractar amb expectativa després de la cirurgia, amb medicaments de fertilitat o amb FIV. Pel que fa al procediment quirúrgic, l'ablació (o fulguració) de l'endometriosi (cremant i vaporitzar les lesions amb un dispositiu elèctric) ha mostrat una alta taxa de recurrència a curt termini després del procediment. El millor procediment quirúrgic amb una taxa molt més baixa de recurrència a curt termini és el d'extirpar (tallar i eliminar) les lesions per complet.
El tractament conservador consisteix en l'extirpació de l'endometri, les adhesions, la resecció dels endometriomes i la restauració de l'anatomia pèlviana tant normal com sigui possible.[84] L'endometrioma a l'ovari de qualsevol mida significativa (aproximadament +2 cm), de vegades diagnosticat com a quist ovàric, s'ha d'eliminar quirúrgicament perquè el tractament hormonal no elimina per complet el quist de l'endometrioma, que pot progressar fins al dolor agut per la ruptura del quist i sagnat intern. La laparoscòpia, a més de ser utilitzada per al diagnòstic, també es pot utilitzar per realitzar cirurgia. Es considera una cirurgia «mínimament invasiva» perquè el cirurgià fa obertures molt petites (incisions) al (o al voltant del) ventre i a la part inferior de la panxa. Un instrument semblant al telescopi (el laparoscopi) es col·loca a través d'una incisió, que permet al metge buscar endometriosi mitjançant una petita càmera unida al laparoscopi. Els instruments petits s'insereixen a través de les incisions per eliminar el teixit de l'endometriosi i les adhesions. Com que les incisions són molt petites, només hi haurà petites cicatrius a la pell després del procediment, i es pot eliminar tota l'endometriosi. Les dones es recuperen de la cirurgia amb més rapidesa i tenen un menor risc d'adhesions.[85]
Entre el 55% i 100% de les dones desenvolupen adhesions després de la cirurgia pelviana,[86] que poden provocar una infertilitat, un dolor crònic abdominal i pelvià, i una cirurgia reoperativa difícil. La suspensió ovàrica temporal de Trehan, una tècnica en què els ovaris són suspesos durant una setmana després de la cirurgia, es pot utilitzar per reduir la incidència d'adhesions després de la cirurgia d'endometriosi.[87][88]
El tractament conservador implica l'extirpació de l'endometriosi i preservar els ovaris i l'úter, molt importants per a les dones que volen concebre, però poden augmentar el risc de recurrència.[89]
La recurrència d'endometriosi després de la cirurgia conservadora s'estima en un 21,5% als 2 anys i en un 40-50% als 5 anys.[90]
Una histerectomia (eliminació de l'úter) es pot utilitzar per tractar l'endometriosi en dones que no desitgen concebre. Tanmateix, això només s'ha de fer quan es combina amb l'extirpació de l'endometriosi per excisió, ja que si l'endometriosi tampoc no s'elimina en el moment de la histerectomia, el dolor pot persistir.[91]
Per a les dones amb un dolor extrem, es pot realitzar una neurectomia presacral, molt poc freqüent, on es tallen els nervis de l'úter. No obstant això, aquesta tècnica gairebé mai s'utilitza a causa de l'alta incidència de complicacions associades, incloent hematoma presacral i problemes irreversibles amb l'orina i el restrenyiment.[91]
Medicaments hormonals[modifica]
- Teràpia hormonal de control de la natalitat: les píndoles anticonceptives redueixen el dolor menstrual associat a l'endometriosi.[92] Poden funcionar reduint o eliminant el flux menstrual i proporcionant un reforç d'estrogen. El control de la natalitat hormonal combinat estrogen-progestagen és el tractament de primera línia per a la majoria de les dones amb endometriosi per la seva capacitat de ser utilitzat durant llargs períodes, pel seu baix cost relatiu i facilitat d'ús, i pel benefici addicional de reduir el risc de càncer d'ovari / endometri.[93]
- Progestagens: la progesterona contraresta l'estrogen i inhibeix el creixement de l'endometri.[94] Aquesta teràpia pot reduir o eliminar la menstruació d'una manera controlada i reversible. Les progestines són variants químiques de progesterona natural. Un exemple de progestina és el dienogest. Tot i que els progestògens solen rebre com a part d'una teràpia hormonal combinada amb l'addició d'estrògens, la teràpia només amb progestàgen pot ser una alternativa acceptable.
- Danazol i gestrinona: són esteroides supressius amb alguna activitat androgènica.[83] Ambdós agents inhibeixen el creixement de l'endometriosi però el seu ús continua sent limitat, ja que poden causar efectes secundaris masculinizants, com ara un creixement excessiu del cabell i canvis de veu.
- Moduladors d'hormones que alliberen gonadotropina (GnRH): aquests fàrmacs inclouen agonistes de GnRH (com leuprorelina) i antagonistes de GnRH (com elagolix), i es creu que funcionen disminuint els nivells d'estrògens.[95] En una revista de Cochrane de 2010, es va publicar que els moduladors de GnRH eren més eficaços per a l'alleugeriment del dolor en endometriosi que cap tractament o placebo, però no eren més efectius que el danazol o el progestagen intrauterí, i tenien més efectes secundaris que el danazol.[95] Una revisió sistemàtica sueca de 2018 va trobar que els moduladors de GnRH tenien efectes semblants per al dolor que el progestagen, però també disminuïa la densitat òssia.[70]
- Inhibidors d'aromatasa: són medicaments que bloquegen la formació d'estrogens i s'han convertit en d'interès per als investigadors que estan tractant l'endometriosi.[96] Exemples d'inhibidors d'aromatasa inclouen anastrozole i letrozole. L'evidència dels inhibidors d'aromatasa és limitada a causa del nombre limitat i la qualitat dels estudis disponibles, tot i que mostren un benefici prometedor en termes de control del dolor.[97]
Altres medicaments[modifica]
- Antiinflamatoris no esteroidal (AINE): s'utilitzen comunament en conjunció amb altres teràpies. Per a casos més greus, es poden utilitzar fàrmacs narcòtics. Les injeccions d'AINE poden ser útils per al dolor greu o l'ús d'AINE oral per a evitar el dolor d'estómac.. Exemples d'AINE inclouen ibuprofèn i naproxén.
- Opioides: els comprimits de sulfat de morfina i altres analgèsics opioides funcionen simulant l'acció de productes químics que redueixen el dolor que es produeixen de manera natural anomenats «endorfines». Hi ha diferents medicaments d'acció prolongada i d'acció curta que es poden utilitzar sols o en combinació per proporcionar un control del dolor adequat.
- Herbes xineses: s'ha informat que després de la cirurgia laparoscòpica, les dones que prenien herbes xineses tenien beneficis comparables amb les dones amb tractaments farmacològics convencionals, tot i que l'article de revista que revisava aquest estudi també va assenyalar que «Els dos assaigs inclosos en aquesta revista tenen una qualitat metodològica deficient. S'ha de interpretar amb cautela. Es necessiten millors assaigs aleatoris controlats de qualitat per investigar un possible paper del CHM (Chinese Herbal Medicine) en el tractament de l'endometriosi».[98]
- Pentoxifil·lina: és un agent immunomodulador que ha estat teoritzat per millorar el dolor i millorar les taxes d'embaràs en dones amb endometriosi. Tanmateix, una revista de Cochrane de 2012 va trobar que no hi havia proves suficients per donar suport a l'efectivitat o la seguretat en aquests usos.[99] Les recomanacions actuals del Congrés americà d'obstetres i ginecòlegs (American Congress of Obstetricians and Gynecologists, ACOG) no inclouen els immunemoduladors, com la pentoxifil·lina, en els protocols de tractament estàndard.[100]
- Antiangiogènics: hi ha una manca d'evidència clínica de la seva eficàcia en la teràpia d'endometriosi.[101] Sota condicions experimentals in vitro i in vivo, els compostos que han demostrat exercir efectes inhibidors en lesions endometriòtiques inclouen inhibidors del factor de creixement, inhibidors endògens d'angiogènesi, anàlegs de fumagilina, estatines, inhibidors de ciclooxigenasa-2, compostos fitoquímics, immunomoduladors, agonistes de dopamina, agonistes de receptors activats pels proliferadors de peroxisomes, progestins, danazol i agonistes de GnRH.[101] Tanmateix, molts d'aquests agents estan associats a efectes secundaris no desitjats i es necessita més recerca. Una teràpia ideal disminuirà la inflamació i els símptomes subjacents sense ser anticonceptius.[102][103]
Encara no s'ha identificat l'efectivitat general de la fisioteràpia manual per tractar l'endometriosi.[104] No hi ha cap evidència que afavoreixi la teràpia nutricional com a eficaç.
Comparació de tractaments[modifica]
Els medicaments i les intervencions quirúrgiques produeixen beneficis aproximadament equivalents al dolor. Es va trobar que la recurrència del dolor era de 44% i 53% amb els medicaments i les intervencions quirúrgiques, respectivament.[30] Cada enfocament té avantatges i desavantatges.[58] La teràpia manual va mostrar una disminució del dolor del 84% de les participants de l'estudi i una millora del 93% en la funció sexual.[105]
Les dades de 2003 sobre la forma en què la medicació era eficaç per alleujar el dolor associat amb l'endometriosi eren limitades.[81] Una revisió sistemàtica sueca del 2018 va trobar una gran quantitat d'estudis, però una manca general d'evidència científica per a la majoria dels tractaments.[70] Només hi havia un estudi de qualitat i rellevància suficient que comparés l'efecte de la cirurgia i la no-cirurgia.[106] Els estudis de cohorts indiquen que la cirurgia és efectiva en la disminució del dolor.[106] La majoria de les complicacions es van produir en casos d'anastomosi intestinal baixa, mentre que el risc de fístula es produïa en casos de cirurgia abdominal o vaginal combinada, i els problemes del tracte urinari eren comuns en la cirurgia intestinal.[106] Es va trobar que l'evidència era insuficient quant a la intervenció quirúrgica.[106]
Els avantatges de la cirurgia demostren l'eficàcia del control del dolor,[107] és més eficaç per a la infertilitat que l'administració de medicaments,[83] proporciona un diagnòstic definitiu,[83] i la cirurgia sovint es pot realitzar com un procediment mínimament invasiu (laparoscòpic) per reduir la morbiditat i minimitzar el risc d'adhesions postoperatòries.[108] S'han realitzat esforços per desenvolupar estratègies efectives per reduir o prevenir adhesions, però la seva formació continua sent un efecte secundari freqüent de la cirurgia abdominal.[86]
Els avantatges de les tècniques de fisioteràpia són el cost reduït, l'absència d'efectes secundaris importants, no interfereix en la fertilitat, i l'augment gairebé sempre la funció sexual.[105] Els inconvenients són que no hi ha estudis a llarg termini sobre el seu ús en el tractament del dolor o la infertilitat relacionada amb l'endometriosi.[105]
Tractament de la infertilitat[modifica]
La cirurgia és més eficaç que la intervenció medicinal per abordar la infertilitat associada a l'endometriosi.[83] La cirurgia intenta eliminar el teixit endometrial i preservar els ovaris sense danyar el teixit normal.[83] Els procediments de fecundació in vitro (FIV) són efectius per millorar la fertilitat en moltes dones amb endometriosi.
Durant el tractament de la fertilitat, el tractament previ molt llarg amb GnRH-agonista té més possibilitats de produir l'embaràs en dones amb endometriosi, en comparació amb el tractament previ curt.[70]
Resultats[modifica]
L'assessorament adequat de dones amb endometriosi requereix atenció a diversos aspectes del trastorn. Té una importància primordial la posada en escena operativa inicial de la malaltia per obtenir informació adequada sobre la base de les futures decisions sobre teràpia. Els símptomes de la dona i el desig de tenir fills dicten la teràpia adequada. No tota la teràpia funciona per a totes les dones. Algunes dones tenen recurrències després de la cirurgia o la pseudomenopausa. En la majoria dels casos, el tractament donarà a les dones un alleugeriment significatiu del dolor pelvià i les ajudarà a aconseguir l'embaràs.[109]
El procés subjacent que causa l'endometriosi pot no cessar després d'una intervenció quirúrgica o mèdica. Els estudis han demostrat que l'endometriosi torna a aparèixer a una velocitat del 20 al 40% dins dels cinc anys posteriors a la cirurgia conservadora,[110] llevat que es realitzi histerectomia o s'arribi a la menopausa. El control de la dona consisteix en exàmens clínics periòdics i en ecografies.
Complicacions[modifica]
Les complicacions de l'endometriosi inclouen cicatrius internes, adhesions, quists pèlvics, endometriomes, ruptura de quists, i obstrucció intestinal i d'uretra resultants d'adhesions pelvianes. La infertilitat associada a l'endometriosi pot estar relacionada amb la formació de cicatrius i distorsions anatòmiques a causa de l'endometriosi.[3]
L'endometriosi ovàrica pot complicar l'embaràs mitjançant la decidualització, abscessos i / o la ruptura.[111]
L'endometriosi toràcica està associada amb pneumotòrax recurrents en períodes menstruals, denominats pneumotòrax catamenial.[112]
Un estudi de 20 anys de 12.000 dones amb endometriosi va trobar que les dones menors de 40 anys que són diagnosticades d'endometriosi tenen 3 vegades més probabilitats de tenir problemes cardíacs que les dones sanes.[113][114]
Produeix poques mortes.[16]
Societat i cultura[modifica]
Tan recent com el 1995, els informes van trobar que més del 50% de les dones amb dolor pelvià crònic no tenien cap causa orgànica, mentre que les dones encara eren considerades mentalment inestables.[115] Els grups d'autoajuda diuen que els professionals retarden el diagnòstic, sovint perquè no ho consideren una possibilitat. Als Estats Units, a partir del 2007, aproximadament el 27% de les dones amb endometriosi havien tingut símptomes almenys sis anys abans de la seva diagnosi.[116]
Els efectes econòmics associats a l'endometriosi són substancials i són similars a altres malalties cròniques, com la malaltia de Crohn, la diabetis o l'artritis reumatoide.[117] Aquesta càrrega econòmica s'atribueix principalment a la incapacitat de treballar de manera constant i predeterminat per la disminució de la qualitat de vida.[117]
Referències[modifica]
- ↑ «Endometriosis: Overview» (en anglès). www.nichd.nih.gov. Arxivat de l'original el 18 maig 2017. [Consulta: 20 maig 2017].
- ↑ «Endometriosis: Condition Information» (en anglès). Arxivat de l'original el 30 abril 2017. [Consulta: 20 maig 2017].
- ↑ 3,00 3,01 3,02 3,03 3,04 3,05 3,06 3,07 3,08 3,09 3,10 3,11 3,12 3,13 3,14 3,15 3,16 3,17 3,18 «Endometriosis» (en anglès), 13-02-2017. Arxivat de l'original el 13 maig 2017. [Consulta: 20 maig 2017].
- ↑ 4,00 4,01 4,02 4,03 4,04 4,05 4,06 4,07 4,08 4,09 4,10 4,11 4,12 4,13 4,14 Bulletti C, Coccia ME, Battistoni S, Borini A «Endometriosis and infertility». Journal of Assisted Reproduction and Genetics, 27, 8, agost 2010, pàg. 441-447. DOI: 10.1007/s10815-010-9436-1. PMC: 2941592. PMID: 20574791.
- ↑ Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M, Raine-Fenning N «The social and psychological impact of endometriosis on women's lives: a critical narrative review». Human Reproduction Update, 19, 6, 01-11-2013, pàg. 625–639. DOI: 10.1093/humupd/dmt027. PMID: 23884896.
- ↑ 6,0 6,1 Vercellini P, Eskenazi B, Consonni D, Somigliana E, Parazzini F, Abbiati A, Fedele L «Oral contraceptives and risk of endometriosis: a systematic review and meta-analysis». Human Reproduction Update, 17, 2, 01-03-2011, pàg. 159–170. DOI: 10.1093/humupd/dmq042. PMID: 20833638.
- ↑ 7,0 7,1 7,2 Brosens, I. Endometriosis: Science and Practice. John Wiley & Sons, 2012, p. 3. ISBN 9781444398496.
- ↑ Batt, Ronald E. A history of endometriosis. Londres: Springer, 2011, p. 13–38. ISBN 978-0-85729-585-9.
- ↑ 9,00 9,01 9,02 9,03 9,04 9,05 9,06 9,07 9,08 9,09 «Endometriosis: ancient disease, ancient treatments». Fertility and Sterility, 98, 6 Suppl, desembre 2012, pàg. S1-62. DOI: 10.1016/j.fertnstert.2012.08.001. PMID: 23084567.
- ↑ «Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015». Lancet, 388, 10053, octubre 2016, pàg. 1545–1602. DOI: 10.1016/S0140-6736(16)31678-6. PMC: 5055577. PMID: 27733282.
- ↑ McGrath, Patrick J.; Stevens, Bonnie J.; Walker, Suellen M.; Zempsky, William T. Oxford Textbook of Paediatric Pain (en anglès). OUP Oxford, 2013, p. 300. ISBN 9780199642656.
- ↑ Nothnick, WB «The emerging use of aromatase inhibitors for endometriosis treatment». Reproductive Biology and Endocrinology, 9, juny 2011, pàg. 87. DOI: 10.1186/1477-7827-9-87. PMC: 3135533. PMID: 21693036.
- ↑ Bulun SE, Zeitoun K, Sasano H, Simpson ER «Aromatase in aging women». Seminars in Reproductive Endocrinology, 17, 4, 1999, pàg. 349–58. DOI: 10.1055/s-2007-1016244. PMID: 10851574.
- ↑ Batt, RE; Mitwally, MF «Endometriosis from thelarche to midteens: pathogenesis and prognosis, prevention and pedagogy». Journal of Pediatric and Adolescent Gynecology, 16, 6, desembre 2003, pàg. 337–47. DOI: 10.1016/j.jpag.2003.09.008. PMID: 14642954.
- ↑ 15,0 15,1 Marsh, EE; Laufer, MR «Endometriosis in premenarcheal girls who do not have an associated obstructive anomaly». Fertility and Sterility, 83, 3, març 2005, pàg. 758–60. DOI: 10.1016/j.fertnstert.2004.08.025. PMID: 15749511.
- ↑ 16,0 16,1 (GBD 2013 Mortality and Causes of Death Collaborators) «Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013». Lancet, 385, 9963, gener 2015, pàg. 117–171. DOI: 10.1016/S0140-6736(14)61682-2. PMC: 4340604. PMID: 25530442.
- ↑ 17,0 17,1 Stratton, P; Berkley, KJ «Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications». Human Reproduction Update, 17, 3, 2011, pàg. 327–346. DOI: 10.1093/humupd/dmq050. PMC: 3072022. PMID: 21106492.
- ↑ 18,0 18,1 18,2 Ballard K, Lane H, Hudelist G, Banerjee S, Wright J «Can specific pain symptoms help in the diagnosis of endometriosis? A cohort study of women with chronic pelvic pain». Fertility and Sterility, 94, 1, juny 2010, pàg. 20–27. DOI: 10.1016/j.fertnstert.2009.01.164. PMID: 19342028.
- ↑ Murray, MT; Pizzorno, J. The Encyclopedia of Natural Medicine (en anglès). 3. Nova York, NY: Simon and Schuster, 2012.
- ↑ Asante, A; Taylor, RN «Endometriosis: the role of neuroangiogenesis». Annual Review of Physiology, 73, 2011, pàg. 163–182. DOI: 10.1146/annurev-physiol-012110-142158. PMID: 21054165.
- ↑ «Treatment of infertility in women with endometriosis». www.uptodate.com. [Consulta: 18 desembre 2017].
- ↑ Wolthuis, Albert M; Meuleman, Christel; Tomassetti, Carla; D'Hooghe, Thomas; de Buck van Overstraeten, Anthony; D'Hoore, André. «Bowel endometriosis: Colorectal surgeon's perspective in a multidisciplinary surgical team (Endometriosi intestinal: perspectiva del cirurgià colorectal en un equip quirúrgic multidisciplinari)» (en anglès). World Journal of Gastroenterology : WJG p. 15616–15623, 14-11-2014. DOI: 10.3748/wjg.v20.i42.15616.
- ↑ Arbique, D; Carter, S; Van Sell, S «Endometriosis can evade diagnosis (L'endometriosi pot evadir el diagnòstic)» (en anglès). Rn, 71.9, setembre 2008, pàg. 28–32; quiz 33. PMID: 18833741.
- ↑ Colette, S; Donnez, J «Are aromatase inhibitors effective in endometriosis treatment? (Són efectius els inhibidors de l'aromatasa en el tractament d'endometriosi?)» (en anglès). Expert Opinion on Investigational Drugs, 20.7, juliol 2011, pàg. 917–931. DOI: 10.1517/13543784.2011.581226. PMID: 21529311.
- ↑ Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG, Chang-Claude J, Hein R, Lurie G, Wilkens LR, Carney ME, Goodman MT, Moysich K, Kjaer SK, Hogdall E, Jensen A, Goode EL, Fridley BL, Larson MC, Schildkraut JM, Palmieri RT, Cramer DW, Terry KL, Vitonis AF, Titus LJ, Ziogas A, Brewster W, Anton-Culver H, Gentry-Maharaj A, Ramus SJ, Anderson AR, Brueggmann D, Fasching PA, Gayther SA, Huntsman DG, Menon U, Ness RB, Pike MC, Risch H, Wu AH, Berchuck A «Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies (Associació entre endometriosi i risc de subtipus histològics de càncer d'ovari: anàlisi conjunt d'estudis de casos i controls)» (en anglès). The Lancet. Oncology, 13.4, abril 2012, pàg. 385–394. DOI: 10.1016/S1470-2045(11)70404-1. PMC: 3664011. PMID: 22361336.
- ↑ Nezhat F. Article by Prof. Farr Nezhat, MD, FACOG, FACS, University of Columbia, May 1, 2012 Arxivat 2012-11-02 a Wayback Machine.
- ↑ Audebert, A «Women with endometriosis: are they different from others? (Dones amb endometriosi: són diferents a les altres?)» (en francès). Gynecologie, Obstetrique & Fertilite, 33, 4, abril 2005, pàg. 239–46. DOI: 10.1016/j.gyobfe.2005.03.010. PMID: 15894210.
- ↑ Rowlands IJ, Nagle CM, Spurdle AB, Webb PM «Gynecological conditions and the risk of endometrial cancer (Condicions ginecològiques i risc de càncer d'endometri)» (en anglès). Gynecologic Oncology, 123.3, pàg. 537–541. DOI: 10.1016/j.ygyno.2011.08.022. PMID: 21925719.
- ↑ 29,0 29,1 29,2 29,3 29,4 29,5 29,6 Fauser BC, Diedrich K, Bouchard P, Domínguez F, Matzuk M, Franks S, Hamamah S, Simón C, Devroey P, Ezcurra D, Howles CM «Contemporary genetic technologies and female reproduction». Human Reproduction Update, 17, 6, 2011, pàg. 829–847. DOI: 10.1093/humupd/dmr033. PMC: 3191938. PMID: 21896560.
- ↑ 30,0 30,1 Kapoor D, Davila W (2005). Endometriosis, Arxivat 2007-11-11 a Wayback Machine. eMedicine.
- ↑ Giudice, LC; Kao, LC «Endometriosis». Lancet, 364, 9447, 2004, pàg. 1789–1799. DOI: 10.1016/S0140-6736(04)17403-5. PMID: 15541453.
- ↑ 32,0 32,1 32,2 32,3 32,4 32,5 32,6 Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT «Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets». Human Reproduction Update, 20, 5, 2014, pàg. 702–716. DOI: 10.1093/humupd/dmu015. PMC: 4132588. PMID: 24676469.
- ↑ Painter JN, Anderson CA, Nyholt DR, Macgregor S, Lin J, Lee SH, Lambert A, Zhao ZZ, Roseman F, Guo Q, Gordon SD, Wallace L, Henders AK, Visscher PM, Kraft P, Martin NG, Morris AP, Treloar SA, Kennedy SH, Missmer SA, Montgomery GW, Zondervan KT «Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis». Nature Genetics, 43, 1, gener 2011, pàg. 51–54. DOI: 10.1038/ng.731. PMC: 3019124. PMID: 21151130.
- ↑ Treloar SA, Wicks J, Nyholt DR, Montgomery GW, Bahlo M, Smith V, Dawson G, Mackay IJ, Weeks DE, Bennett ST, Carey A, Ewen-White KR, Duffy DL, O'connor DT, Barlow DH, Martin NG, Kennedy SH «Genomewide linkage study in 1,176 affected sister pair families identifies a significant susceptibility locus for endometriosis on chromosome 10q26». American Journal of Human Genetics, 77, 3, setembre 2005, pàg. 365–76. DOI: 10.1086/432960. PMC: 1226203. PMID: 16080113.
- ↑ Sinaii N, Plumb K, Cotton L, Lambert A, Kennedy S, Zondervan K, Stratton P «Differences in characteristics among 1,000 women with endometriosis based on extent of disease». Fertility and Sterility, 89, 3, març 2008, pàg. 538–545. DOI: 10.1016/j.fertnstert.2007.03.069. PMC: 2939902. PMID: 17498711.
- ↑ 36,0 36,1 Giudice, LC «Clinical practice. Endometriosis». The New England Journal of Medicine, 362, 25, juny 2010, pàg. 2389–2398. DOI: 10.1056/NEJMcp1000274. PMC: 3108065. PMID: 20573927.
- ↑ Treloar SA, Bell TA, Nagle CM, Purdie DM, Green AC «Early menstrual characteristics associated with subsequent diagnosis of endometriosis». American Journal of Obstetrics and Gynecology, 202, 6, juny 2010, pàg. 534.e1-536. DOI: 10.1016/j.ajog.2009.10.857. PMID: 20022587.
- ↑ Nnoaham KE, Webster P, Kumbang J, Kennedy SH, Zondervan KT «Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies». Fertility and Sterility, 98, 3, setembre 2012, pàg. 702–712.e6. DOI: 10.1016/j.fertnstert.2012.05.035. PMC: 3502866. PMID: 22728052.
- ↑ Anger, DL; Foster, WG «The link between environmental toxicant exposure and endometriosis». Frontiers in Bioscience, 13, gener 2008, pàg. 1578–1593. DOI: 10.2741/2782. PMID: 17981650.
- ↑ Guo, SW «The link between exposure to dioxin and endometriosis: a critical reappraisal of primate data». Gynecologic and Obstetric Investigation, 57, 3, 2004, pàg. 157–173. DOI: 10.1159/000076374. PMID: 14739528.
- ↑ «Reassessing the evidence for the link between dioxin and endometriosis: from molecular biology to clinical epidemiology». Molecular Human Reproduction, 15, 10, octubre 2009, pàg. 609–624. DOI: 10.1093/molehr/gap075. PMID: 19744969.
- ↑ Rier, S; Foster, WG «Environmental dioxins and endometriosis». Toxicological Sciences, 70, 2, desembre 2002, pàg. 161–170. DOI: 10.1093/toxsci/70.2.161. PMID: 12441361.
- ↑ van der Linden, PJ «Theories on the pathogenesis of endometriosis». Human Reproduction, 11 Suppl 3, novembre 1996, pàg. 53–65. DOI: 10.1093/humrep/11.suppl_3.53. PMID: 9147102.
- ↑ Pinkert, TC; Catlow, CE; Straus, R «Endometriosis of the urinary bladder in a man with prostatic carcinoma». Cancer, 43, 4, abril 1979, pàg. 1562–1567. DOI: 10.1002/1097-0142(197904)43:4%3C1562::aid-cncr2820430451%3E3.0.co;2-w. PMID: 445352.
- ↑ Signorile PG, Baldi F, Bussani R, D'Armiento M, De Falco M, Baldi A «Ectopic endometrium in human foetuses is a common event and sustains the theory of müllerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer». Journal of Experimental & Clinical Cancer Research, 28, abril 2009, pàg. 49. DOI: 10.1186/1756-9966-28-49. PMC: 2671494. PMID: 19358700.
- ↑ Mok-Lin EY, Wolfberg A, Hollinquist H, Laufer MR «Endometriosis in a patient with Mayer-Rokitansky-Küster-Hauser syndrome and complete uterine agenesis: evidence to support the theory of coelomic metaplasia». Journal of Pediatric and Adolescent Gynecology, 23, 1, febrer 2010, pàg. e35-37. DOI: 10.1016/j.jpag.2009.02.010. PMID: 19589710.
- ↑ Thibodeau LL, Prioleau GR, Manuelidis EE, Merino MJ, Heafner MD «Cerebral endometriosis. Case report». Journal of Neurosurgery, 66, 4, abril 1987, pàg. 609–610. DOI: 10.3171/jns.1987.66.4.0609. PMID: 3559727.
- ↑ Rodman, MH; Jones, CW «Catamenial hemoptysis due to bronchial endometriosis». The New England Journal of Medicine, 266, 16, abril 1962, pàg. 805–808. DOI: 10.1056/nejm196204192661604. PMID: 14493132.
- ↑ «Endopædia». Arxivat de l'original el 2018-05-13. [Consulta: 3 juliol 2018].
- ↑ Gleicher N, el-Roeiy A, Confino E, Friberg J «Is endometriosis an autoimmune disease?». Obstetrics and Gynecology, 70, 1, juliol 1987, pàg. 115–122. PMID: 3110710.
- ↑ Capellino S, Montagna P, Villaggio B, Sulli A, Soldano S, Ferrero S, Remorgida V, Cutolo M «Role of estrogens in inflammatory response: expression of estrogen receptors in peritoneal fluid macrophages from endometriosis». Annals of the New York Academy of Sciences, 1069, juny 2006, pàg. 263–267. DOI: 10.1196/annals.1351.024. PMID: 16855153.
- ↑ 52,0 52,1 Young VJ, Brown JK, Saunders PT, Horne AW «The role of the peritoneum in the pathogenesis of endometriosis». Human Reproduction Update, 19, 5, 2013, pàg. 558–569. DOI: 10.1093/humupd/dmt024. PMID: 23720497.
- ↑ Redwine, DB «Was Sampson wrong?». Fertility and Sterility, 78, 4, octubre 2002, pàg. 686–693. DOI: 10.1016/S0015-0282(02)03329-0. PMID: 12372441.
- ↑ Hufnagel D, Li F, Cosar E, Krikun G, Taylor HS «The Role of Stem Cells in the Etiology and Pathophysiology of Endometriosis». Seminars in Reproductive Medicine, 33, 5, setembre 2015, pàg. 333–340. DOI: 10.1055/s-0035-1564609. PMC: 4986990. PMID: 26375413.
- ↑ Bruner-Tran KL, Yeaman GR, Crispens MA, Igarashi TM, Osteen KG «Dioxin may promote inflammation-related development of endometriosis». Fertility and Sterility, 89, 5 Suppl, maig 2008, pàg. 1287–1298. DOI: 10.1016/j.fertnstert.2008.02.102. PMC: 2430157. PMID: 18394613.
- ↑ «Nickel Allergy Is a Risk Factor for Endometriosis: An 11-Year Population-Based Nested Case-Control Study». PLOS One, 10, 10, 2015, pàg. e0139388. DOI: 10.1371/journal.pone.0139388. PMC: 4594920. PMID: 26439741.
- ↑ Signorile PG, Baldi F, Bussani R, D'Armiento M, De Falco M, Baldi A «Ectopic endometrium in human foetuses is a common event and sustains the theory of müllerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer». Journal of Experimental & Clinical Cancer Research, 28, abril 2009, pàg. 49. DOI: 10.1186/1756-9966-28-49. PMC: 2671494. PMID: 19358700.
- ↑ 58,0 58,1 «Diagnosis and Treatment of Endometriosis». American Academy of Family Physicians, 15-10-1999. Arxivat de l'original el 2011-06-06. [Consulta: 26 juliol 2011].
- ↑ Laschke MW, Giebels C, Menger MD «Vasculogenesis: a new piece of the endometriosis puzzle». Human Reproduction Update, 17, 5, 2011, pàg. 628–636. DOI: 10.1093/humupd/dmr023. PMID: 21586449.
- ↑ Morotti M, Vincent K, Brawn J, Zondervan KT, Becker CM «Peripheral changes in endometriosis-associated pain». Human Reproduction Update, 20, 5, 2014, pàg. 717–736. DOI: 10.1093/humupd/dmu021. PMC: 4337970. PMID: 24859987.
- ↑ Yuk JS, Park EJ, Seo YS, Kim HJ, Kwon SY, Park WI «Graves Disease Is Associated With Endometriosis: A 3-Year Population-Based Cross-Sectional Study». Medicine, 95, 10, març 2016, pàg. e2975. DOI: 10.1097/MD.0000000000002975. PMC: 4998884. PMID: 26962803.
- ↑ Giudice, LC; Kao, LC «Endometriosis». Lancet, 364, 9447, 2004, pàg. 1789–1799. DOI: 10.1016/S0140-6736(04)17403-5. PMID: 15541453.
- ↑ Weed, JC; Ray, JE «Endometriosis of the bowel». Obstetrics and Gynecology, 69, 5, maig 1987, pàg. 727–730. PMID: 3574800.
- ↑ Uzunçakmak C, Güldaş A, Ozçam H, Dinç K «Scar endometriosis: a case report of this uncommon entity and review of the literature». Case Reports in Obstetrics and Gynecology, 2013, 2013, pàg. 386783. DOI: 10.1155/2013/386783. PMC: 3665185. PMID: 23762683.
- ↑ Dwivedi AJ, Agrawal SN, Silva YJ «Abdominal wall endometriomas». Digestive Diseases and Sciences, 47, 2, febrer 2002, pàg. 456–461. DOI: 10.1023/a:1013711314870. PMID: 11855568.
- ↑ Kaunitz, A; Di Sant'Agnese, PA «Needle tract endometriosis: an unusual complication of amniocentesis». Obstetrics and Gynecology, 54, 6, desembre 1979, pàg. 753–755. PMID: 160025.
- ↑ Koger KE, Shatney CH, Hodge K, McClenathan JH «Surgical scar endometrioma». Surgery, Gynecology & Obstetrics, 177, 3, setembre 1993, pàg. 243–246. PMID: 8356497.
- ↑ Daly S. «Endometrioma/Endometriosis». e-Medecine. WebMD, 18-10-2004. Arxivat de l'original el 6 febrer 2007. [Consulta: 19 desembre 2006].
- ↑ «Getting diagnosed with endometriosis | Endometriosis UK» (en anglès). www.endometriosis-uk.org. Arxivat de l'original el 2021-03-01. [Consulta: 13 juny 2018].
- ↑ 70,0 70,1 70,2 70,3 70,4 70,5 70,6 «Endometriosis – Diagnosis, treatment and patient experiences» (en anglès). Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU), 04-05-2018. [Consulta: 13 juny 2018].
- ↑ 71,0 71,1 Fang, Jing; Piessens, Sofie «A step-by-step guide to sonographic evaluation of deep infiltrating endometriosis» (en anglès). Sonography, 5, 2, 04-06-2018, pàg. 67–75. DOI: 10.1002/sono.12149. ISSN: 2202-8323.
- ↑ Office on Women's Health, U.S. Department of Health and Human Services. (16 July 2012). Endometriosis Fact Sheet. Retrieved from Womenshealth.gov «Archived copy». Arxivat de l'original el 2015-07-03. [Consulta: 11 juliol 2015].
- ↑ Nisolle M, Paindaveine B, Bourdon A, Berlière M, Casanas-Roux F, Donnez J «Histologic study of peritoneal endometriosis in infertile women». Fertility and Sterility, 53, 6, juny 1990, pàg. 984–988. PMID: 2351237.
- ↑ «Treatment of pelvic pain associated with endometriosis: a committee opinion». Fertility and Sterility, 101, 4, abril 2014, pàg. 927–935. DOI: 10.1016/j.fertnstert.2014.02.012. PMID: 24630080.
- ↑ American Society For Reproductive M, «Revised American Society for Reproductive Medicine classification of endometriosis: 1996». Fertility and Sterility, 67, 5, maig 1997, pàg. 817–821. DOI: 10.1016/S0015-0282(97)81391-X. PMID: 9130884.
- ↑ 76,0 76,1 76,2 76,3 76,4 May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM «Peripheral biomarkers of endometriosis: a systematic review». Human Reproduction Update, 16, 6, 2010, pàg. 651–674. DOI: 10.1093/humupd/dmq009. PMC: 2953938. PMID: 20462942.
- ↑ 77,0 77,1 Hirsch M, Duffy J, Davis CJ, Nieves Plana M, Khan KS «Diagnostic accuracy of cancer antigen 125 for endometriosis: a systematic review and meta-analysis». Bjog, 123, 11, octubre 2016, pàg. 1761–1768. DOI: 10.1111/1471-0528.14055. PMID: 27173590.
- ↑ May KE, Villar J, Kirtley S, Kennedy SH, Becker CM «Endometrial alterations in endometriosis: a systematic review of putative biomarkers». Human Reproduction Update, 17, 5, 2011, pàg. 637–653. DOI: 10.1093/humupd/dmr013. PMID: 21672902.
- ↑ «Archived copy». Arxivat de l'original el 2013-05-02. [Consulta: 18 juliol 2013].
- ↑ Bourdel N, Alves J, Pickering G, Ramilo I, Roman H, Canis M «Systematic review of endometriosis pain assessment: how to choose a scale?». Human Reproduction Update, 21, 1, 2014, pàg. 136–152. DOI: 10.1093/humupd/dmu046. PMID: 25180023.
- ↑ 81,0 81,1 «What are the treatments for endometriosis». Eunice Kennedy Shriver National Institute of Child Health and Human Development. Arxivat de l'original el 3 agost 2013. [Consulta: 20 agost 2013].
- ↑ Moen MH, Rees M, Brincat M, Erel T, Gambacciani M, Lambrinoudaki I, Schenck-Gustafsson K, Tremollieres F, Vujovic S, Rozenberg S «EMAS position statement: Managing the menopause in women with a past history of endometriosis». Maturitas, 67, 1, setembre 2010, pàg. 94–97. DOI: 10.1016/j.maturitas.2010.04.018. PMID: 20627430.
- ↑ 83,0 83,1 83,2 83,3 83,4 83,5 Wellbery, C «Diagnosis and treatment of endometriosis». American Family Physician, 60, 6, octubre 1999, pàg. 1753–1762, 1767–1768. PMID: 10537390.
- ↑ Speroff, L; Glass, RH; Kase, NG. Clinical Gynecologic Endocrinology and Infertility. 6. Lippincott Willimas Wilkins, 1999, p. 1057. ISBN 0-683-30379-1.
- ↑ «Endometriosis and Infertility: Can Surgery Help?». American Society for Reproductive Medicine, 2008. Arxivat de l'original el 2010-10-11. [Consulta: 31 octubre 2010].
- ↑ 86,0 86,1 Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL «Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management». Digestive Surgery, 18, 4, 2001, pàg. 260–273. DOI: 10.1159/000050149. PMID: 11528133.
- ↑ Trehan, AK «Temporary ovarian suspension». Gynaecological Endoscopy, 11, 1, 2002, pàg. 309–314. DOI: 10.1046/j.1365-2508.2002.00520.x.
- ↑ Abuzeid MI, Ashraf M, Shamma FN «Temporary ovarian suspension at laparoscopy for prevention of adhesions». The Journal of the American Association of Gynecologic Laparoscopists, 9, 1, febrer 2002, pàg. 98–102. PMID: 11821616.
- ↑ Namnoum AB, Hickman TN, Goodman SB, Gehlbach DL, Rock JA «Incidence of symptom recurrence after hysterectomy for endometriosis». Fertility and Sterility, 64, 5, novembre 1995, pàg. 898–902. PMID: 7589631.
- ↑ Guo, SW «Recurrence of endometriosis and its control». Human Reproduction Update, 15, 4, 2009, pàg. 441–461. DOI: 10.1093/humupd/dmp007. PMID: 19279046.
- ↑ 91,0 91,1 Johnson, NP; Hummelshoj, L «Consensus on current management of endometriosis». Human Reproduction, 28, 6, juny 2013, pàg. 1552–1568. DOI: 10.1093/humrep/det050. PMID: 23528916.
- ↑ Zorbas, KA; Economopoulos, KP; Vlahos, NF «Continuous versus cyclic oral contraceptives for the treatment of endometriosis: a systematic review». Archives of Gynecology and Obstetrics, 292, 1, juliol 2015, pàg. 37–43. DOI: 10.1007/s00404-015-3641-1. PMID: 25644508.
- ↑ «Endometriosis: Treatment of pelvic pain». [Consulta: 18 desembre 2017].
- ↑ Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S «Role of nuclear progesterone receptor isoforms in uterine pathophysiology». Human Reproduction Update, 21, 2, 2014, pàg. 155–173. DOI: 10.1093/humupd/dmu056. PMC: 4366574. PMID: 25406186.
- ↑ 95,0 95,1 Brown, J; Pan, A; Hart, RJ «Gonadotrophin-releasing hormone analogues for pain associated with endometriosis». The Cochrane Database of Systematic Reviews, 12, desembre 2010, pàg. CD008475. DOI: 10.1002/14651858.CD008475.pub2. PMID: 21154398.
- ↑ Attar, E; Bulun, SE «Aromatase inhibitors: the next generation of therapeutics for endometriosis?». Fertility and Sterility, 85, 5, maig 2006, pàg. 1307–1318. DOI: 10.1016/j.fertnstert.2005.09.064. PMID: 16647373.
- ↑ Nawathe, A; Patwardhan, S; Yates, D; Harrison, GR; Khan, KS «Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis». Bjog, 115, 7, juny 2008, pàg. 818–822. DOI: 10.1111/j.1471-0528.2008.01740.x. PMID: 18485158.
- ↑ Flower A, Liu JP, Lewith G, Little P, Li Q «Chinese herbal medicine for endometriosis». The Cochrane Database of Systematic Reviews, 5, maig 2012, pàg. CD006568. DOI: 10.1002/14651858.CD006568.pub3. PMID: 22592712.
- ↑ Lu D, Song H, Li Y, Clarke J, Shi G «Pentoxifylline for endometriosis». The Cochrane Database of Systematic Reviews, 1, gener 2012, pàg. CD007677. DOI: 10.1002/14651858.CD007677.pub3. PMID: 22258970.
- ↑ «Practice bulletin no. 114: management of endometriosis». Obstetrics and Gynecology, 116, 1, juliol 2010, pàg. 223–236. DOI: 10.1097/AOG.0b013e3181e8b073. PMID: 20567196.
- ↑ 101,0 101,1 Laschke, MW; Menger, MD «Anti-angiogenic treatment strategies for the therapy of endometriosis». Human Reproduction Update, 18, 6, 2012, pàg. 682–702. DOI: 10.1093/humupd/dms026. PMID: 22718320.
- ↑ Canny, GO; Lessey, BA «The role of lipoxin A4 in endometrial biology and endometriosis». Mucosal Immunology, 6, 3, maig 2013, pàg. 439–450. DOI: 10.1038/mi.2013.9. PMC: 4062302. PMID: 23485944.
- ↑ Streuli I, de Ziegler D, Santulli P, Marcellin L, Borghese B, Batteux F, Chapron C «An update on the pharmacological management of endometriosis». Expert Opinion on Pharmacotherapy, 14, 3, febrer 2013, pàg. 291–305. DOI: 10.1517/14656566.2013.767334. PMID: 23356536.
- ↑ Valiani M, Ghasemi N, Bahadoran P, Heshmat R «The effects of massage therapy on dysmenorrhea caused by endometriosis». Iranian Journal of Nursing and Midwifery Research, 15, 4, 2010, pàg. 167–171. PMC: 3093183. PMID: 21589790.
- ↑ 105,0 105,1 105,2 Wurn BF, Wurn LJ, Patterson K, King CR, Scharf ES «Decreasing dyspareunia and dysmenorrhea in women with endometriosis via a manual physical therapy: Results from two independent studies». Journal of Endometriosis and Pelvic Pain Disorders, 3, 2011, pàg. 188–196. Arxivat de l'original el 2013-10-29. DOI: 10.5301/JE.2012.9088 [Consulta: 22 setembre 2018].
- ↑ 106,0 106,1 106,2 106,3 «Endometrios – diagnostik, behandling och bemötande» (en suec) p. 121. Statens beredning för medicinsk och social utvärdering (SBU); Swedish Agency for Health Technology Assessment and Assessment of Social Services. [Consulta: 13 juny 2018].
- ↑ Kaiser A, Kopf A, Gericke C, Bartley J, Mechsner S «The influence of peritoneal endometriotic lesions on the generation of endometriosis-related pain and pain reduction after surgical excision». Archives of Gynecology and Obstetrics, 280, 3, setembre 2009, pàg. 369–373. DOI: 10.1007/s00404-008-0921-z. PMID: 19148660.
- ↑ Radosa MP, Bernardi TS, Georgiev I, Diebolder H, Camara O, Runnebaum IB «Coagulation versus excision of primary superficial endometriosis: a 2-year follow-up». European Journal of Obstetrics, Gynecology, and Reproductive Biology, 150, 2, juny 2010, pàg. 195–198. DOI: 10.1016/j.ejogrb.2010.02.022. PMID: 20303642.
- ↑ Memarzadeh S, Muse KN, Fox, MD. «Endometriosis». Differential Diagnosis and Treatment of endometriosis.. Armenian Health Network, Health.am, 21-09-2006. Arxivat de l'original el 31 gener 2007. [Consulta: 19 desembre 2006].
- ↑ «Recurrent Endometriosis: Surgical Management». The Cleveland Clinic, 07-01-2010. Arxivat de l'original el 2010-05-01. [Consulta: 31 octubre 2010].
- ↑ Ueda Y, Enomoto T, Miyatake T, Fujita M, Yamamoto R, Kanagawa T, Shimizu H, Kimura T «A retrospective analysis of ovarian endometriosis during pregnancy». Fertility and Sterility, 94, 1, juny 2010, pàg. 78–84. DOI: 10.1016/j.fertnstert.2009.02.092. PMID: 19356751.
- ↑ Visouli AN, Zarogoulidis K, Kougioumtzi I, Huang H, Li Q, Dryllis G, Kioumis I, Pitsiou G, Machairiotis N, Katsikogiannis N, Papaiwannou A, Lampaki S, Zaric B, Branislav P, Porpodis K, Zarogoulidis P «Catamenial pneumothorax». Journal of Thoracic Disease, 6, Suppl 4, octubre 2014, pàg. S448-S460. DOI: 10.3978/j.issn.2072-1439.2014.08.49. PMC: 4203986. PMID: 25337402.
- ↑ Wise, Jacqui «Women with endometriosis show higher risk for heart disease» (en anglès). BMJ, 353, 01-04-2016, pàg. i1851. DOI: 10.1136/bmj.i1851. ISSN: 1756-1833. PMID: 27036948.
- ↑ «Women with endometriosis at higher risk for heart disease | American Heart Association» (en anglès). [Consulta: 3 juliol 2018].
- ↑ Gomel, V; Taylor, PJ. {{{títol}}}. St. Louis, MO: Mosby, 1995.
- ↑ Overton C, Davis C, McMillan L, Shaw RW. {{{títol}}}. 3. Londres: Informa Healthcare, 2007, p. 9–10.
- ↑ 117,0 117,1 Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, Brodszky V, Canis M, Colombo GL, DeLeire T, Falcone T, Graham B, Halis G, Horne A, Kanj O, Kjer JJ, Kristensen J, Lebovic D, Mueller M, Vigano P, Wullschleger M, D'Hooghe T «The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres». Human Reproduction, 27, 5, maig 2012, pàg. 1292–1299. DOI: 10.1093/humrep/des073. PMID: 22422778.
Enllaços externs[modifica]
| A Wikimedia Commons hi ha contingut multimèdia relatiu a: Endometriosi |
- «Endometriosi». Canal Salut. Generalitat de Catalunya. [Consulta: 18 febrer 2020].
- Endometriosi a Curlie (anglès)
- Endometriosis fact sheet al Office on Womens Healt (anglès)
